This is a valid set because all four quantum numbers have permitted values. This quantum number set describes an electron located in the third energy shell, in the #3d# subshell, in one of the five #3d# orbitals, that has spin-down.Given an array of numbers, arrange them in a way that yields the largest value. For example, if the given numbers are {54, 546, 548, 60}, the arrangement 6054854654 gives the largest value. # possible integer from given array. # of integers... # custom comparator to sort according.Simply sorting the array in descending order and considering the sorted order is not possible here as the sorted array {75, 68, 21, 12, 10, 7} will result in the For two numbers, X and Y, the custom comparator function will not compare X and Y with each other, but it compares XY with YX, and the...Numbers 1, 2, 3,., n are known as natural numbers. This program takes the value of n and finds the sum of first n natural numbers. Enter the value of n(should be a positive integer): 5 Sum of first n natural numbers is: 15.This is because for a given principal quantum number, n, the corresponding set of angular quantum numbers, l, is equivalent to a range between 0 and (n-1). For n=2, (n-1) is equal to 1, so the range is 0 and 1. The magnetic quantum number, m, can be determined by a range from -l to +l. So for this...
Arrange given numbers to form the biggest number | Set 1
This means that the angular quantum number for a principal quantum number of 2 is equivalent to 0 0, 1 0, 1, 2 0, 1, 2, 3" in Chemistry if you're in doubt about the correctness of the answers or there's no answer, then try to use the smart search and find answers to the similar questions.How can I choose some numbers from a given list so that their sum is a certain given number? Please clarify: do you want to find 2 numbers which sum to the desired number, or the smallest size set that sums to it? What happens if there are multiple sets which sum to the desired number...Therefore there are 2 values for l. m on the other hand , depends on l where for n=2 , we found that l = 2. firstly before answering the question i just wanted to brush up your memories like there are four theories regarding quantum number .the first one is azimutual quantum number that describe the...Also, set $s[0] = 0$. Let us now iterate over the index $r = 1 \ldots n$, and learn how to quickly find the optimal $l$ for each current value $r$, at To do this, we need to learn how to solve the following subproblem: given the number $x$, and we need to check whether there is a subarray of array $a...

Find the largest number possible from a given set of numbers
For a given principal quantum number or n, the corresponding angular quantum number or l is equivalent to a range between 0 and (n-1). Top powerful money spell call +27673406922 get you richness.To all Countries If you need to get rich as fast as possible contact Priest Mandela for th … e...This is because for a given principal quantum number, n, the corresponding set of angular quantum numbers, l, is equivalent to a range between 0 and (n-1). For n=2, (n-1) is equal to 1, so the range is 0 and 1. The magnetic quantum number, m, can be determined by a range from -l to +l. So for this...Part B. Give the values for n, l, and ml for each orbital in the 5d subshell. Enter your answer as sets of values of n, l, and ml for different orbitals separated by SubmitMy AnswersGive Up. Incorrect; Try Again; 6 attempts remaining; no points deducted. Your answer does not have the correct number of...For l= 2, what are the possible values of ml?For n=4, l=0, 1, 2, 3; for l=2, ml=-2, -1, 0, +1, +2. CHEM 1110 - Spring 2013. THE 4 QUANTUM NUMBERS with practice and key. 12 pages. The energies of subshells within a given principal energy level always increase.Number of Proper Subsets of the Set For example: 1. If A {1, 3, 5}, then write all the possible subsets of A. Find their numbers. Solution: The subset of A containing no elements
Find a solution on your query ✅ "Which set of numbers gives the correct possible values of l for n = 2? 0 0, 1 0, 1, 2 0, 1, 2, 3 ..." in 📘 Chemistry if you are in doubt about the correctness of the answers or there is not any answer, then try to use the sensible seek and find solutions to the an identical questions.
Search for Other AnswersMatematica, La Maestra Corregge "Male" Il Compito All'Alunno

Dedicated To Ashley & Iris -

Development Of An ACR Appropriateness Criteria Database

NEWS AND REVIEWS OF WORK DIRECTLY RELATED TO BAKER STREET

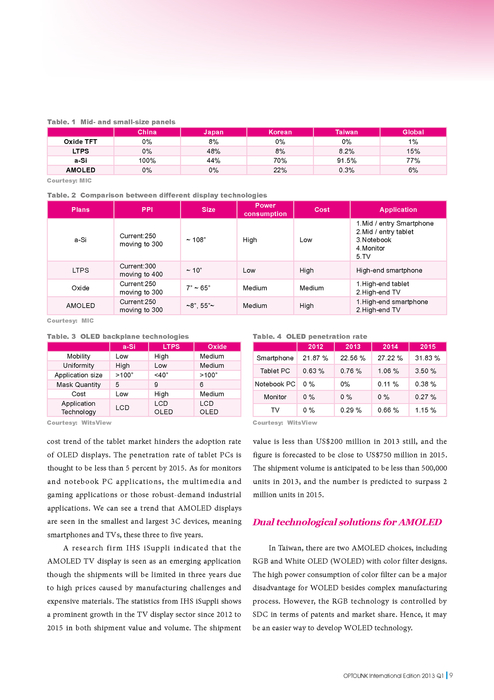
Http://www.gogofinder.com.tw/books/pida/1/ OPTOLINK 2013

FREEDOMFIGHTERS FOR AMERICA - THIS ORGANIZATIONEXPOSING

Europe - ThinEbook E-books

Http://www.gogofinder.com.tw/books/pida/1/ OPTOLINK 2013

Http://www.gogofinder.com.tw/books/pida/1/ OPTOLINK 2013
FREEDOMFIGHTERS FOR AMERICA - THIS ORGANIZATIONEXPOSING

General Science Praxis -- Chemistry Flashcards | Quizlet

Http://www.gogofinder.com.tw/books/pida/1/ OPTOLINK 2013







Tidak ada komentar:
Posting Komentar